







Stimpod NMS460 Features
- Long-term relief from chronic pain
- Restores optimal nerve functionality
- Fast onset of action
- No side-effects
- Online training for clinicians
- Portable and easy to use
- Supported by multiple clinical trials

Stimpod NMS460 Features
- Long term relief from chronic pain
- Restores natural nerve functionality
- Fast onset of action
- No side-effects
- Specialist training for DCs, MDs and PTs
- Portable and easy to use
- Backed by multiple clinical trials

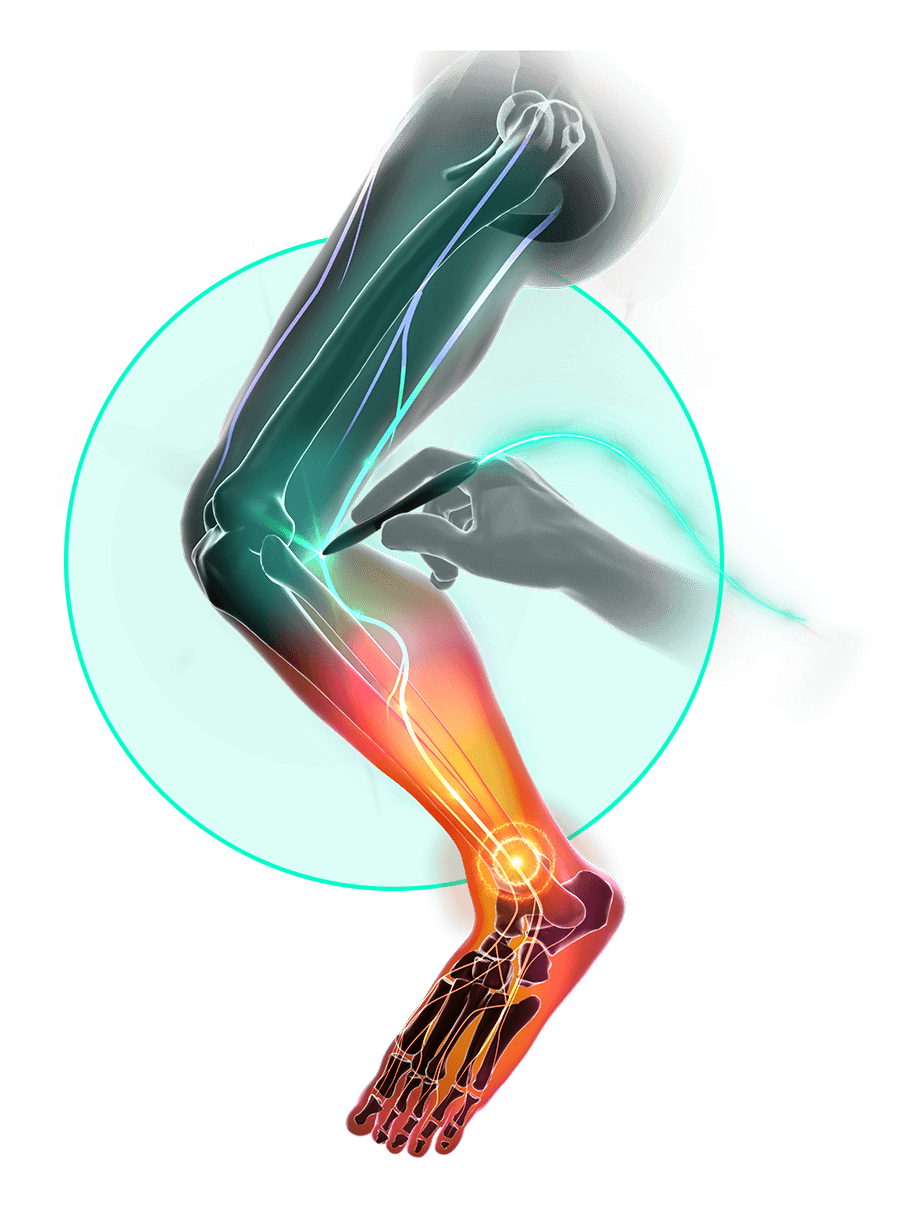
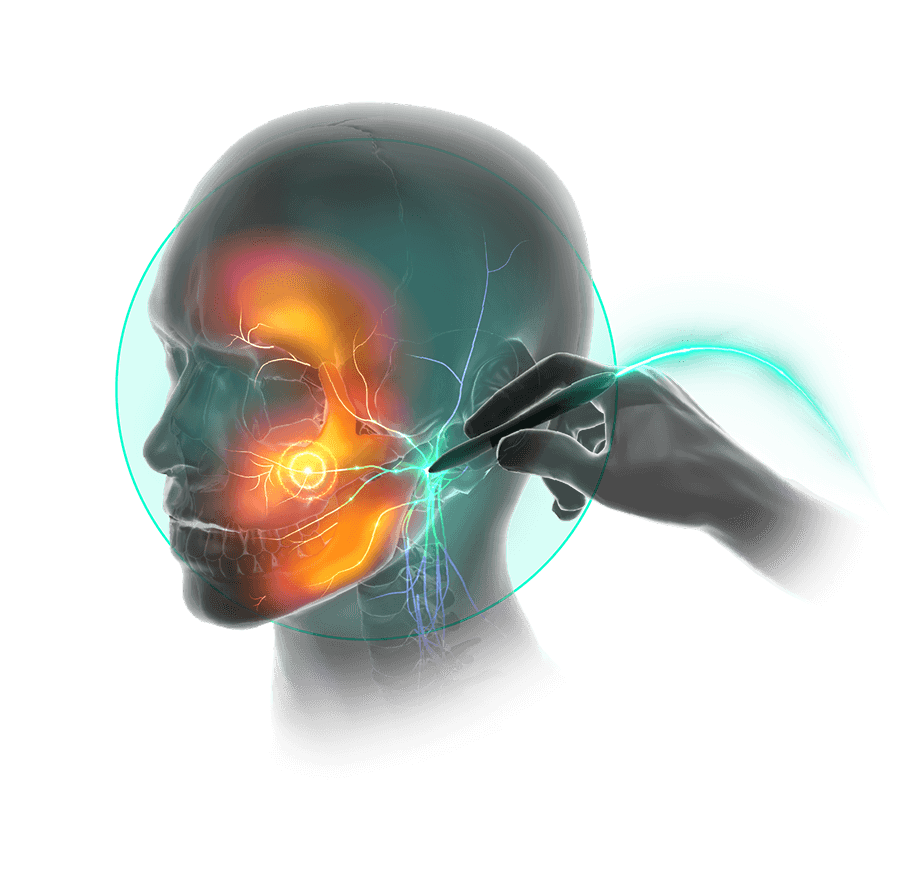
The Stimpod’s patented waveform is delivered transcutaneously and affects the cellular metabolic activity of a neuropathic nerve.
The induced electromagnetic field propagates as short bursts and travels along the axons to the spinal dorsal horn, restoring intra-neural communication pathways and natural nerve functionality.
Nerve locating technology allows you to find the affected nerve through the skin. Accurate placement of the treatment probe is confirmed by muscle twitches for motor nerves and patient feedback for sensory nerves.
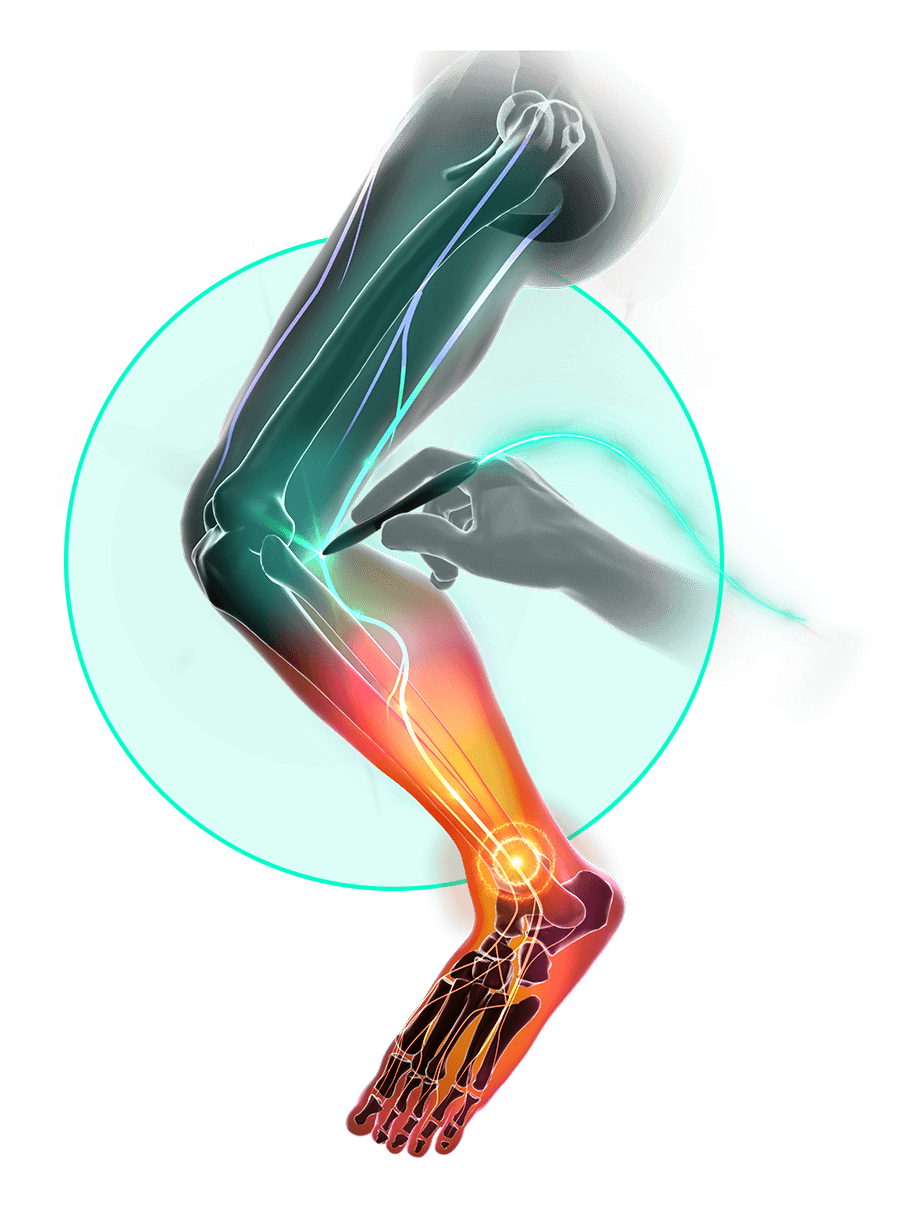
The Stimpod’s patented waveform is delivered transcutaneously and effects the cellular metabolic activity of a neuropathic nerve.
The induced electromagnetic field propagates as short bursts and travels along the axons to the spinal dorsal horn, restoring intra-neural communication pathways and natural nerve functionality.
Nerve locating technology allows you to find the affected nerve through the skin. Accurate placement of the treatment probe is confirmed by muscle twitching for motor nerves and patient feedback for sensory nerves.
Backed by multiple clinical trials and research
Clinical Trials Include:
- Trigeminal Neuralgia
- Chronic Headaches
- Diabetic Neuropathy
- Intractable Pain


Backed by multiple clinical trials and research
Clinical Trials Include:
- Trigeminal Neuralgia
- Chronic Headaches
- Diabetic Neuropathy
- Intractable Pain

What our doctors say
Expand the scope of
your practice
The Stimpod NMS460 has been a game changer in my practice. There is not a DC or PT, LAC, Neurologist, Orthopedist, etc. that should not include this as part of their patient assessment, treatment and follow up.
Dr. Edythe Heus
Doctor of Chiropractic
See the Stimpod in Action
Watch how easy it is to setup the Stimpod and start treating your patients with our Quick Start Guide video.

The Stimpod is the World's First
Non-Invasive Pulsed Radio Frequency Treatment System
The Stimpod is indicated for:
- The symptomatic relief and management
of chronic intractable pain - An adjunctive treatment in the management of post-surgical pain
- An adjunctive treatment for post-traumatic,
acute pain problems - An adjunctive treatment for pain
control due to rehabilitation


The Stimpod is the World's First
Non-Invasive Pulsed Radio Frequency Treatment System
The Stimpod is indicated for:
- The symptomatic relief and management of chronic intractable pain
- An adjunctive treatment in the management of post-surgical pain
- An adjunctive treatment for post-traumatic, acute
pain problems - An adjunctive treatment for pain control due to rehabilitation



What the patients say
What is non-invasive PRF?
Algiamed’s electromagnetic therapies are designed to restore the natural functionality of nerve cells using a non-invasive approach. The therapy is administered with a pen-like stimulation probe on the skin surface and elicits a light electrical sensation.
Our patented waveform delivers brief bursts of pulse stimulation triggering a long-lasting recovery through a metabolic biochemical cascade which targets the root cause of neurologically related diseases.
The induced electromagnetic field oscillates at frequencies in the range of the radio spectrum. It propagates as short bursts through the skin to the nerves and travels along the axons to the spinal dorsal horn, restoring the intra-neural communication pathways and the natural nerve functionality.
The short-burst electromagnetic stimulation in the radiofrequency range is what classifies Algiamed’s non-invasive electromagnetic therapies as PRF.
CLEARED AND CERTIFIED

The STIMPOD NMS460 is cleared by the United States Food and Drug Administration and it complies with the essential requirements of the relevant European health, safety, and environmental protection legislation (CE certified).

CORPORATE OFFICES
Algiamed Technologies
2025 Willingdon Ave #900
Burnaby, BC V5C 0J3
Canada


